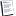
Going to do a little Holley Carb math to explain how Lbs of Air Mass relates to Cubic Feet of Air.
First I will post up some assumptions:
ṁ = 1 Lb/Minute ṁ = Air Mass in Lbs per Minute
R = 1545.34896 R = an engineering constant
Temp 1 = 85.31 T1 85.31 degrees F Air Temp
T0 = 459.67 T0 = A Temperature Constant
Ta = 544.98 Ta = Temperature in Rankin
z = 1 z = an engineering constant
Pa = 13.948
P1 = 0
MWair = 28.9644 Molecular Weight Of Air
Q = 14.4766528950463 CFM
Q1 = 0.0690767408219194 lbs/cu ft Air Mass
So how did we get there?
Using a simplified formula: Assume 1 lb of air mass
1 lb air mass x 1545.34896 x [85.31 x 459.67] x 1 ------------- (you can plug in 70 degrees where 85.31 is)
------------------------------------------------------------
28.9644 x 144 x 13.948 --------------- (You can plug in 14.7 psi where 13.948 is)
equals 842184.2762
-----------------
58175.34497
or 14.4766529 cfm ----------------------- The new result would be 13.35019399 cfm using 70 and 14.7.
So really if you copy down the formula above and plug in say 70 degrees F vs the 85.31 F Temp number and you plug in 14.7 PSI vs the 13.948 PSI number your new cfm per pound of air mass would be 13.35019399 cfm.
Pretty easy calculation if you use the simplified version. You only change two numbers Temp and Pressure
Tom V.
__________________
"Engineers do stuff for reasons" Tom Vaught
Despite small distractions, there are those who will go Forward, Learning, Sharing Knowledge, Doing what they can to help others move forward.
Last edited by Tom Vaught; 01-30-2018 at 10:38 PM.
|